
Three applications of the above expressions are as follows: In practice, the external pressure or temperature needs to be infinitesimally different from that of the system to ensure the appropriate direction of change. In reversible heating, the temperature of the external heater is matched to the changing temperature of the system at all stages of the heating: there is thermal equilibrium throughout. In a reversible expansion, the external pressure is matched to the changing pressure of the system at all stages of the expansion: there is mechanical equilibrium throughout. In a reversible process, the system and its surroundings are in equilibrium. A reversible process is one that changes direction when an external variable, such as pressure or temperature, is changed by an infinitesimal amount. The definition refers to the reversible transfer of energy as heat. It is commonly demonstrated that S is indeed a state function by using the Carnot cycle, as explained in textbooks. It is far from obvious that the definition in (1) implies that S is a state function, or equivalently that d S is an exact differential (one with a definite integral that is independent of the path of integration). 1Įntropy is a state function that is, it has a value that depends only upon the current state of the system and is independent of how that state was prepared.
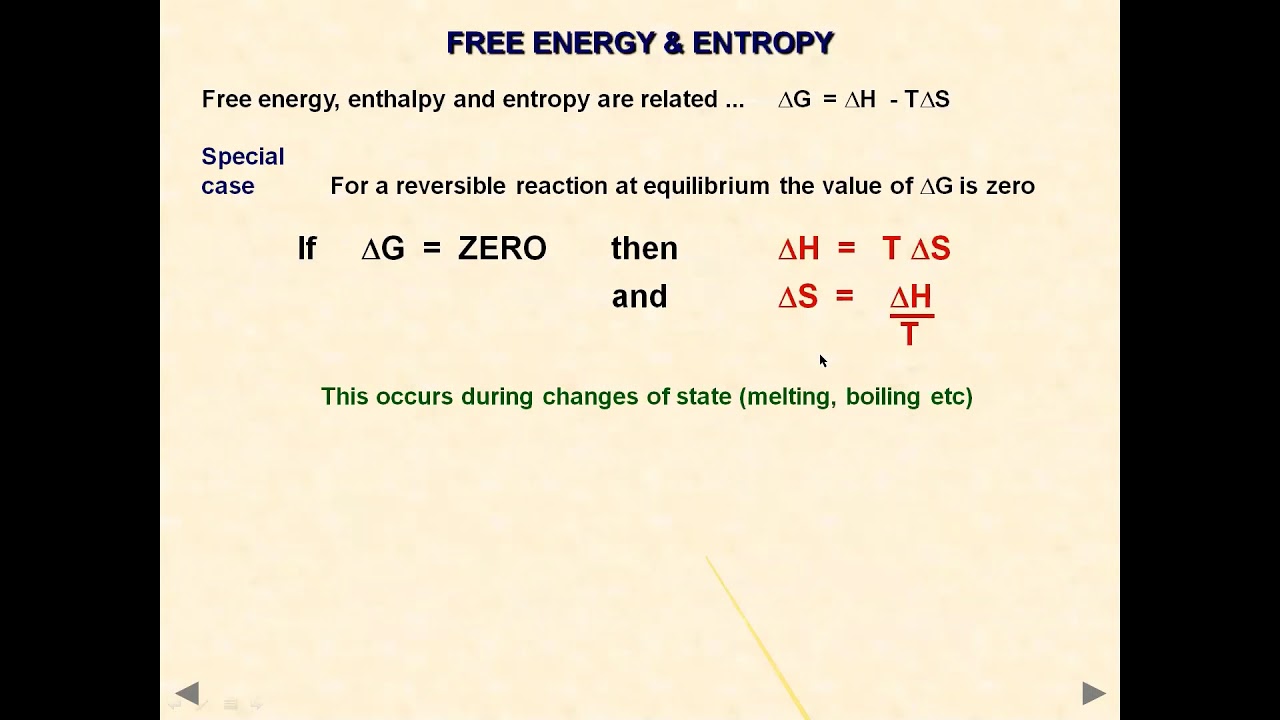
With energy (and, by implication, heat) in joules (J) and temperature in kelvins (K), the units of entropy are joules per kelvin (J K -1). Where q rev is the total energy transferred reversibly during the change of state.


 0 kommentar(er)
0 kommentar(er)
